More Average Atomic Mass Worksheet: A Comprehensive Guide delves into the fascinating realm of atomic mass, providing a comprehensive overview of its significance and practical applications. This worksheet serves as an invaluable resource for students and professionals seeking to deepen their understanding of this fundamental chemical concept.
The worksheet guides users through the step-by-step process of calculating average atomic mass, exploring the concept of weighted averages, and examining the diverse types of isotopes that contribute to an element’s average atomic mass. It also delves into real-world applications of average atomic mass in chemistry, physics, and materials science.
1. Introduction
Atomic mass, often referred to as atomic weight, is a fundamental property of an element. It represents the average mass of all the naturally occurring isotopes of that element, taking into account their relative abundances.
The purpose of this worksheet is to provide a comprehensive understanding of the concept of average atomic mass and the methods used to calculate it. It will cover the types of isotopes, the concept of weighted average, and the applications of average atomic mass in various scientific fields.
2. Calculating Average Atomic Mass
To calculate the average atomic mass of an element, the following steps are typically followed:
- Identify all the naturally occurring isotopes of the element and their respective isotopic abundances.
- Multiply the atomic mass of each isotope by its isotopic abundance expressed as a fraction or percentage.
- Sum the products obtained in step 2.
- Divide the sum by 100 to obtain the average atomic mass.
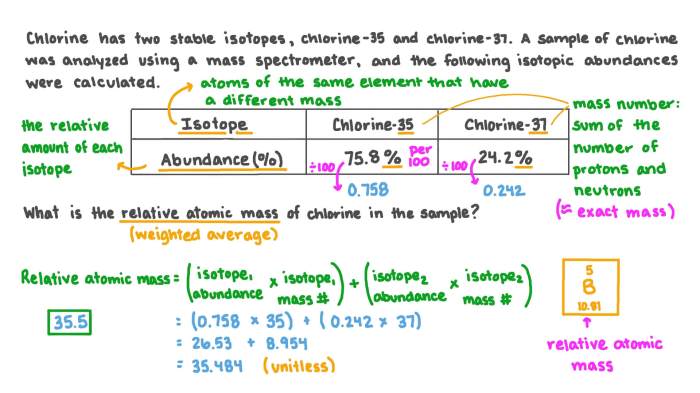
For example, the average atomic mass of chlorine can be calculated as follows:
- Isotope 35Cl has an atomic mass of 34.96885 amu and an isotopic abundance of 75.77%.
- Isotope 37Cl has an atomic mass of 36.96590 amu and an isotopic abundance of 24.23%.
Average atomic mass = (34.96885 amu x 0.7577) + (36.96590 amu x 0.2423) = 35.453 amu
3. Types of Isotopes
Isotopes are atoms of the same element that have the same atomic number but different neutron numbers. This difference in neutron number results in different atomic masses for isotopes of the same element.
Isotopes can be classified into two main types:
- Stable isotopesare those that do not undergo radioactive decay. They are found in nature and are responsible for the element’s average atomic mass.
- Radioactive isotopesare those that undergo radioactive decay, transforming into different elements over time. They are found in nature but are often less abundant than stable isotopes.
The neutron number plays a crucial role in determining the stability of an isotope. Isotopes with a neutron-to-proton ratio that is too high or too low are often unstable and undergo radioactive decay.
4. Weighted Average
Weighted average is a statistical concept that is used to calculate the average of a set of values, taking into account the relative importance or weight of each value.
In the context of calculating average atomic mass, the isotopic abundances of the different isotopes represent the weights. The atomic mass of each isotope is multiplied by its isotopic abundance, and the products are summed. The sum is then divided by 100 to obtain the average atomic mass.
For example, consider an element with two isotopes:
- Isotope A has an atomic mass of 10 amu and an isotopic abundance of 60%.
- Isotope B has an atomic mass of 12 amu and an isotopic abundance of 40%.
Weighted average atomic mass = (10 amu x 0.60) + (12 amu x 0.40) = 11.2 amu
5. Applications of Average Atomic Mass: More Average Atomic Mass Worksheet
Average atomic mass is a fundamental property of an element that finds applications in various scientific fields, including:
- Chemistry:Average atomic mass is used to calculate the molar mass of compounds, which is essential for stoichiometric calculations and understanding the composition of substances.
- Physics:Average atomic mass is used in nuclear physics to calculate the mass of atomic nuclei and study nuclear reactions.
- Materials science:Average atomic mass is used in materials science to design and develop new materials with specific properties.
For example, in chemistry, the average atomic mass of chlorine is used to calculate the molar mass of sodium chloride (NaCl). The molar mass of NaCl is 58.44 g/mol, which is the sum of the average atomic masses of sodium (22.99 amu) and chlorine (35.45 amu).
Popular Questions
What is atomic mass?
Atomic mass is the weighted average mass of all the isotopes of an element, taking into account their relative abundances.
How do you calculate average atomic mass?
Average atomic mass is calculated by multiplying the mass of each isotope by its abundance and summing the products.
What are isotopes?
Isotopes are atoms of the same element with different numbers of neutrons.