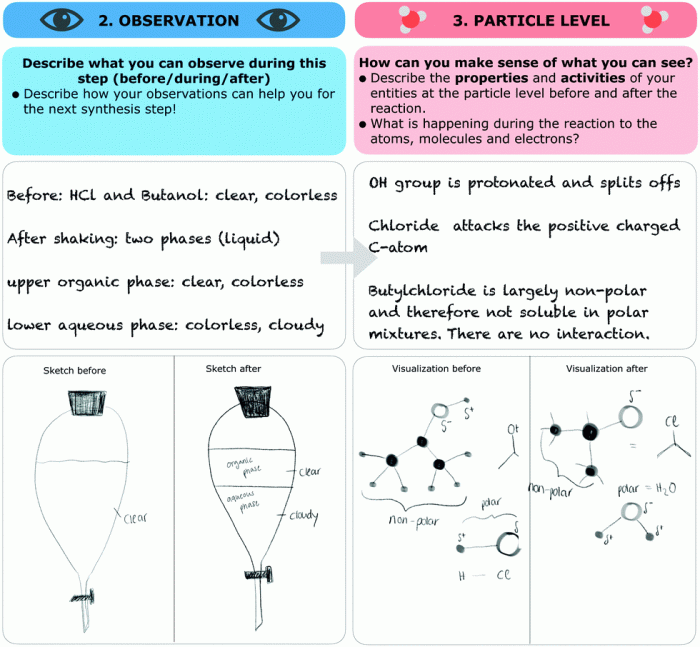
Gizmo polarity and intermolecular forces orchestrate a captivating dance at the molecular level, shaping the properties and behavior of matter. Understanding this intricate interplay unlocks a treasure trove of insights into the world around us.
Gizmo polarity refers to the uneven distribution of electrical charge within a molecule, creating a molecular dipole. These dipoles, like tiny magnets, interact with each other through intermolecular forces, influencing the physical properties of substances.
Gizmo Polarity
Gizmo polarity refers to the separation of electrical charges within a gizmo molecule, resulting in a positive end and a negative end. This polarity plays a significant role in determining the intermolecular forces between gizmos.
Polar gizmos have a permanent dipole moment due to the uneven distribution of electrons. This polarity can be caused by differences in electronegativity between atoms within the gizmo molecule or by the presence of lone pairs of electrons.
Types of Intermolecular Forces
- Dipole-dipole forcesoccur between polar gizmos with permanent dipole moments. These forces are weaker than covalent bonds but stronger than van der Waals forces.
- Hydrogen bondingis a particularly strong type of dipole-dipole force that occurs when a hydrogen atom is bonded to a highly electronegative atom such as oxygen, nitrogen, or fluorine.
- Van der Waals forcesare weak attractive forces that exist between all gizmos, regardless of their polarity. These forces include London dispersion forces, which arise from the temporary fluctuations in electron distribution, and permanent dipole-induced dipole forces, which occur when a permanent dipole in one gizmo induces a dipole in a neighboring gizmo.
Intermolecular Forces and Gizmo Properties, Gizmo polarity and intermolecular forces
The strength of intermolecular forces directly influences the physical properties of gizmos.
- Boiling point: Gizmos with stronger intermolecular forces have higher boiling points because more energy is required to overcome these forces and separate the gizmos.
- Melting point: Gizmos with stronger intermolecular forces have higher melting points because more energy is required to break the intermolecular forces and allow the gizmos to move past each other.
- Solubility: Gizmos with stronger intermolecular forces are less soluble in nonpolar solvents because the intermolecular forces between the gizmos are stronger than the intermolecular forces between the gizmos and the solvent molecules.
Applications of Gizmo Polarity and Intermolecular Forces
Understanding gizmo polarity and intermolecular forces has numerous applications in various fields.
- Drug design: Polarity and intermolecular forces play a crucial role in the design of drugs that can interact with specific biological targets.
- Materials science: The properties of materials, such as their strength and durability, can be tailored by controlling the polarity and intermolecular forces between the constituent gizmos.
- Separation techniques: Polarity and intermolecular forces are used in separation techniques such as chromatography and electrophoresis to separate gizmos based on their differences in polarity and intermolecular interactions.
Commonly Asked Questions: Gizmo Polarity And Intermolecular Forces
What is gizmo polarity?
Gizmo polarity is the uneven distribution of electrical charge within a molecule, resulting in a molecular dipole.
How do intermolecular forces affect physical properties?
Intermolecular forces influence properties such as boiling point, melting point, and solubility by determining the strength of the interactions between molecules.
What are the applications of gizmo polarity and intermolecular forces?
Understanding these concepts finds applications in fields like drug design, material science, and nanotechnology, enabling the development of new technologies and materials with tailored properties.