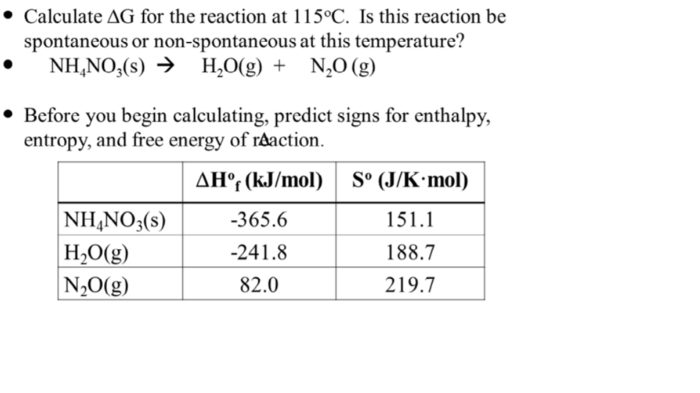
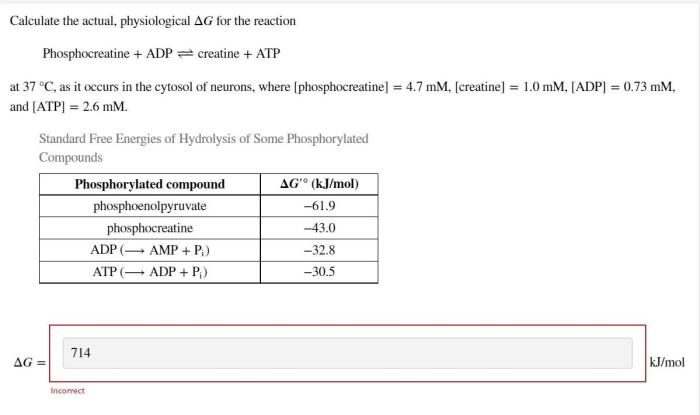
Calculate the actual physiological delta G for the reaction: Unveiling the significance of this intricate concept in cellular processes. This article delves into the depths of physiological delta G, providing a comprehensive guide to its calculation, influencing factors, and practical applications.
Physiological delta G, a fundamental thermodynamic parameter, plays a pivotal role in understanding the energetics of biochemical reactions within living systems. Its accurate calculation is essential for deciphering the intricate dance of cellular metabolism and unraveling the mysteries of life’s intricate processes.
Understanding the Physiological Delta G

The physiological delta G, or free energy change, is a measure of the energy available to drive a chemical reaction in a biological system. It is a key concept in biochemistry and cell biology, as it determines the feasibility and direction of many cellular processes.
The physiological delta G is different from the standard delta G, which is calculated under standard conditions (1 M concentration, 298 K, pH 7). The physiological delta G takes into account the actual concentrations of reactants and products in a cell, as well as the temperature and pH of the cellular environment.
Calculating the Actual Physiological Delta G, Calculate the actual physiological delta g for the reaction
The physiological delta G can be calculated using the following equation:
ΔG = ΔG°’ + RT ln([P]/[R])
where:
- ΔG is the physiological delta G
- ΔG°’ is the standard delta G
- R is the gas constant (8.314 J/mol K)
- T is the temperature in Kelvin
- [P] is the concentration of the products
- [R] is the concentration of the reactants
Methods for Determining Physiological Delta G
There are several experimental methods that can be used to measure the physiological delta G. These methods include:
- Isothermal calorimetry: This method measures the heat released or absorbed by a reaction, which can be used to calculate the delta G.
- Fluorescence spectroscopy: This method measures the fluorescence of a molecule that is involved in the reaction, which can be used to calculate the delta G.
- NMR spectroscopy: This method measures the nuclear magnetic resonance of a molecule that is involved in the reaction, which can be used to calculate the delta G.
Each of these methods has its own advantages and disadvantages, and the choice of method will depend on the specific reaction being studied.
Factors Affecting Physiological Delta G
The physiological delta G can be affected by a number of factors, including:
- Temperature: The delta G of a reaction is typically more negative at higher temperatures.
- pH: The delta G of a reaction can be affected by the pH of the environment.
- Ionic strength: The delta G of a reaction can be affected by the ionic strength of the environment.
- Concentration of reactants and products: The delta G of a reaction is affected by the concentrations of the reactants and products.
Applications of Physiological Delta G
The physiological delta G is a useful tool for understanding and predicting the behavior of chemical reactions in biological systems. It is used in a variety of applications, including:
- Drug design: The physiological delta G can be used to design drugs that are more likely to be effective in the body.
- Biotechnology: The physiological delta G can be used to design and optimize biotechnological processes.
- Medicine: The physiological delta G can be used to understand and diagnose diseases.
Illustrating Physiological Delta G with Tables
The following table summarizes the variables involved in calculating the physiological delta G:
| Variable | Description |
|---|---|
| ΔG | Physiological delta G |
| ΔG°’ | Standard delta G |
| R | Gas constant (8.314 J/mol K) |
| T | Temperature in Kelvin |
| [P] | Concentration of the products |
| [R] | Concentration of the reactants |
The following table compares different methods for determining physiological delta G:
| Method | Advantages | Disadvantages |
|---|---|---|
| Isothermal calorimetry | Accurate | Time-consuming |
| Fluorescence spectroscopy | Fast | Less accurate |
| NMR spectroscopy | Non-invasive | Expensive |
Quick FAQs: Calculate The Actual Physiological Delta G For The Reaction
What is physiological delta G?
Physiological delta G is the change in free energy under physiological conditions, taking into account factors such as temperature, pH, and the presence of ions.
Why is calculating the actual physiological delta G important?
Calculating the actual physiological delta G allows researchers to determine the feasibility and direction of biochemical reactions under physiological conditions, providing insights into cellular metabolism and energy balance.
What are the methods for determining physiological delta G?
Physiological delta G can be determined experimentally using methods such as calorimetry, enzyme assays, and electrochemical measurements.