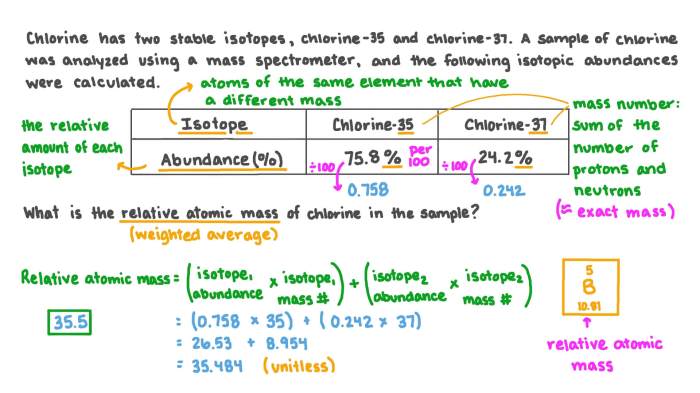
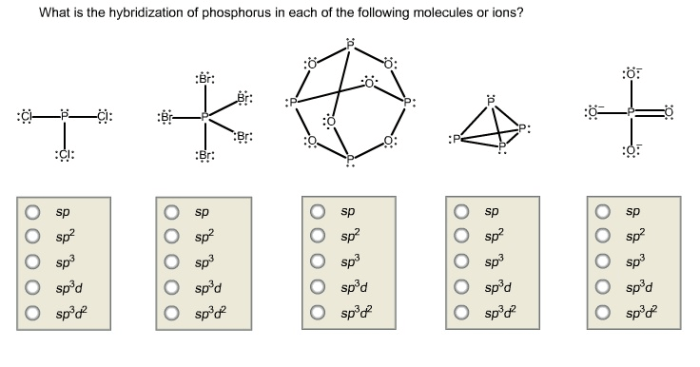
Determine the hybridization of phosphorus in each molecule or ion. This intriguing topic embarks us on a journey into the realm of chemistry, where we unravel the complexities of atomic structures and their profound influence on molecular properties.
Hybridization, a cornerstone of chemical bonding, plays a pivotal role in determining the geometry, reactivity, and overall behavior of molecules. In this discourse, we delve into the intricacies of phosphorus hybridization, exploring how it shapes the characteristics of various phosphorus-containing compounds.
Definition of Hybridization: Determine The Hybridization Of Phosphorus In Each Molecule Or Ion.
Hybridization is a concept in chemistry that explains the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. These hybrid orbitals are used to form chemical bonds and determine the molecular geometry and properties of compounds.
There are different types of hybridization, including:
- sp hybridization: Two atomic orbitals (one s and one p) combine to form two sp hybrid orbitals that are linear in shape.
- sp 2hybridization: Three atomic orbitals (one s and two p) combine to form three sp 2hybrid orbitals that are trigonal planar in shape.
- sp 3hybridization: Four atomic orbitals (one s and three p) combine to form four sp 3hybrid orbitals that are tetrahedral in shape.
Hybridization of Phosphorus

Phosphorus is a versatile element that can exhibit different hybridization states depending on its molecular environment.
In most compounds, phosphorus adopts sp 3hybridization. This occurs when phosphorus is bonded to four other atoms, such as in the case of phosphorus pentachloride (PCl 5) and phosphoric acid (H 3PO 4).
However, phosphorus can also exhibit sp 2hybridization when it is bonded to three other atoms, as in the case of phosphorus trichloride (PCl 3). In some cases, phosphorus can even adopt sp hybridization, as in the case of phosphorus dichloride (PCl 2).
Molecular Geometry and Hybridization
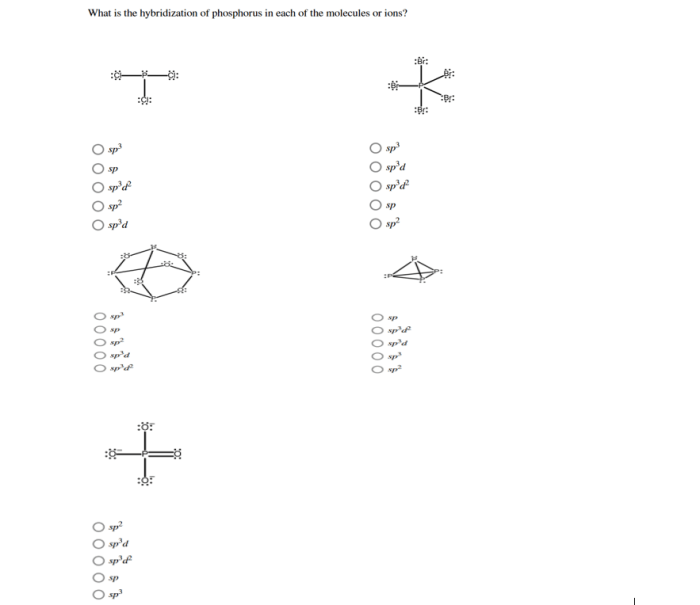
The hybridization of an atom directly influences the molecular geometry of the compound it forms. The hybrid orbitals of an atom determine the number and orientation of the electron pairs around the atom, which in turn determines the shape of the molecule.
For example, an atom with sp 3hybrid orbitals will form four bonds and have a tetrahedral molecular geometry. An atom with sp 2hybrid orbitals will form three bonds and have a trigonal planar molecular geometry. An atom with sp hybrid orbitals will form two bonds and have a linear molecular geometry.
Examples of Phosphorus Hybridization
| Molecule/Ion | Hybridization | Molecular Geometry | Bond Angles |
|---|---|---|---|
| PCl5 | sp3 | Trigonal bipyramidal | 90°, 120° |
| PCl3 | sp2 | Trigonal pyramidal | 109.5° |
| PCl2 | sp | Linear | 180° |
Methods for Determining Hybridization
The hybridization of an atom can be determined using various experimental and theoretical methods:
- X-ray crystallography: This technique involves analyzing the diffraction of X-rays by a crystal lattice to determine the positions and bonding of atoms.
- Electron diffraction: This technique involves analyzing the diffraction of electrons by a gas sample to determine the molecular structure and hybridization.
- NMR spectroscopy: This technique involves using nuclear magnetic resonance to determine the chemical environment and hybridization of atoms.
- Computational chemistry: This technique involves using computer programs to calculate the electronic structure and hybridization of molecules.
Applications of Hybridization in Chemistry
Understanding hybridization has numerous applications in chemistry, including:
- Predicting molecular geometry: Hybridization can be used to predict the molecular geometry of compounds, which is important for understanding their properties and reactivity.
- Designing new materials: Hybridization can be used to design new materials with specific properties, such as strength, conductivity, and optical properties.
- Drug design: Hybridization can be used to design drugs that interact with specific biological targets, which is important for developing new treatments for diseases.
Popular Questions
What is hybridization?
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies.
How does hybridization affect molecular geometry?
Hybridization determines the arrangement of atoms in a molecule, influencing its shape and bond angles.
What factors influence the hybridization of phosphorus?
The number of valence electrons, the electronegativity of the bonded atoms, and the steric effects of neighboring atoms all play a role in phosphorus hybridization.