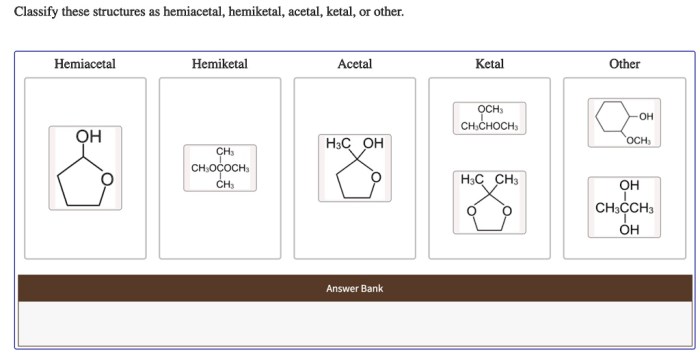
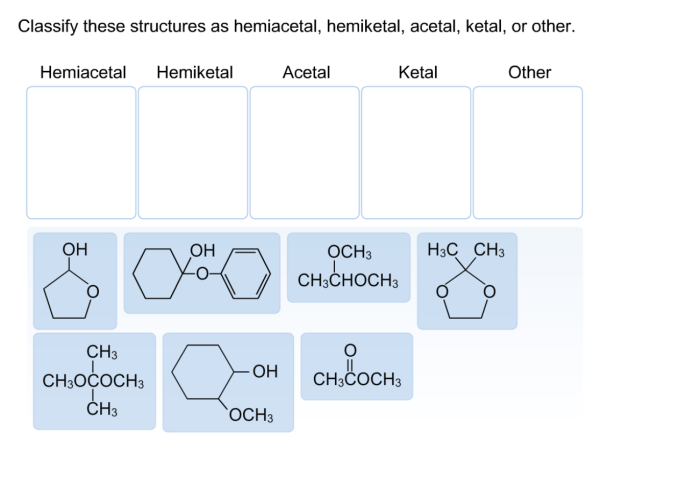
Classify these structures as hemiacetal hemiketal acetal ketal or other – In the realm of organic chemistry, classifying chemical structures as hemiacetals, hemiketals, acetals, ketals, or others is a crucial aspect of understanding their properties and reactivity. These functional groups play significant roles in various chemical processes and possess distinct characteristics that differentiate them from one another.
This comprehensive guide delves into the identification, classification, and chemical reactions of these structures, providing a thorough understanding of their significance in organic chemistry.
Types of Structures: Classify These Structures As Hemiacetal Hemiketal Acetal Ketal Or Other
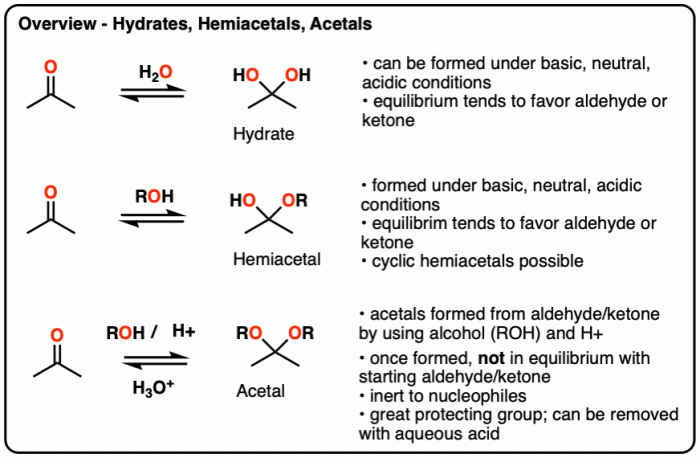
Acetals, ketals, hemiacetals, and hemiketals are organic compounds that contain functional groups with both ether and alcohol moieties. These structures play significant roles in various chemical reactions and have diverse applications in organic chemistry and other fields.
Hemiacetals, Classify these structures as hemiacetal hemiketal acetal ketal or other
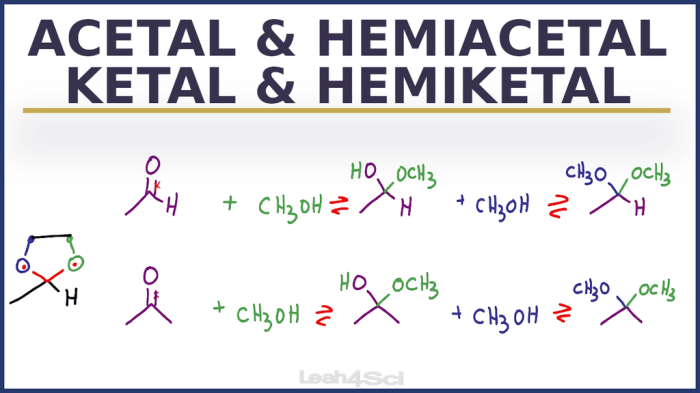
Hemiacetals are compounds that contain a hydroxyl group (-OH) and an alkoxy group (-OR) attached to the same carbon atom. They are formed by the reaction of an aldehyde or ketone with an alcohol in the presence of an acid catalyst.
Hemiacetals are typically unstable and can easily undergo further reactions to form acetals or undergo hydrolysis to regenerate the aldehyde or ketone and alcohol.
Hemiketals
Hemiketals are similar to hemiacetals, but they are formed by the reaction of a ketone with two equivalents of an alcohol. Hemiketals have two alkoxy groups (-OR) and one hydroxyl group (-OH) attached to the same carbon atom. They are also unstable and can undergo further reactions to form ketals or undergo hydrolysis to regenerate the ketone and alcohol.
Acetals
Acetals are compounds that contain two alkoxy groups (-OR) attached to the same carbon atom. They are formed by the reaction of an aldehyde or ketone with two equivalents of an alcohol in the presence of an acid catalyst. Acetals are more stable than hemiacetals and hemiketals and are less likely to undergo hydrolysis.
Ketals
Ketals are similar to acetals, but they are formed by the reaction of a ketone with two equivalents of an alcohol. Ketals have two alkoxy groups (-OR) and one hydroxyl group (-OH) attached to the same carbon atom. They are more stable than acetals and are less likely to undergo hydrolysis.
FAQ Resource
What are the key differences between hemiacetals and hemiketals?
Hemiacetals are formed by the reaction of an aldehyde or ketone with one equivalent of an alcohol, while hemiketals are formed by the reaction of an aldehyde or ketone with two equivalents of an alcohol. Hemiacetals have a hydroxyl group (-OH) and an ether group (-OR), while hemiketals have two ether groups (-OR).
How can acetals be distinguished from ketals?
Acetals are formed by the reaction of an aldehyde with two equivalents of an alcohol, while ketals are formed by the reaction of a ketone with two equivalents of an alcohol. Acetals have two ether groups (-OR) attached to the same carbon atom, while ketals have two ether groups attached to different carbon atoms.
What are the common applications of acetals and ketals?
Acetals and ketals are widely used as protecting groups for carbonyl compounds. They can also be used as solvents, plasticizers, and fragrances.